Fuel gas blending: Follow the H2 signal
The transition of the larger energy industry away from fossil fuels has taken some interesting directions, one being the move toward hydrogen (H2) as a means of storing energy. One of the lowest carbon intensity pathways to H2 is electrolysis powered by renewable electricity, such as wind farms, solar photovoltaic panels or hydroelectric power. When surplus power is available, it can be directed to an electrolyzer to produce green H2 (and oxygen) from water.
H2 can be used as a feedstock in various chemical processes (e.g., hydrotreating in a refinery or ammonia production) or as a fuel across several types of power or heat generating equipment, including boilers, turbines and engines, among others. In this latter function, it can be burned in its pure form or blended with natural gas to reduce overall carbon emissions. The blending approach is more common because H2, in its pure form, has very different burning characteristics compared to natural gas. This relationship will be examined in greater detail later in the article.
So, is natural gas blending a worthwhile use for H2? One must keep in mind the relationship between emissions reduction and percent volume of H2 in natural gas is non-linear. For example, when blended at 20% with natural gas, carbon dioxide (CO2) emissions are reduced by 7% (FIG. 1).
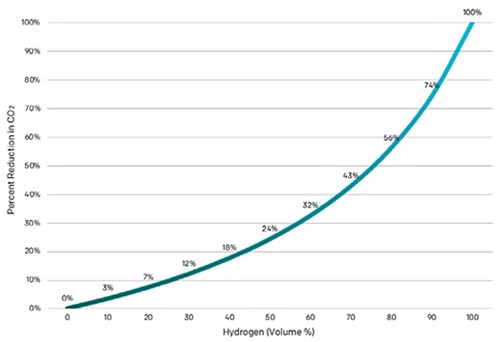
FIG. 1. CO2 emissions reductions, when blending H2 with natural gas, are proportional to the blending ratio.
With H2 hubs on the rise around the U.S.—largely in response to sizeable subsidies distributed by the U.S. Department of Energy because of the Infrastructure Investment and Jobs Act of 2021—many low-carbon intensity H2 projects are incentivized to move forward. In some of these cases, there is no long-term offtake agreement or clear demand yet in place. Existing gas turbine operators can take advantage of this moment by being “H2-ready,” providing the end-user demand, or benefitting from surplus H2 in the event its production is tied to intermittent renewable energy like solar and wind.
In some cases, it may be practical to blend H2 into the local natural gas distribution network via an agreement with local utilities, but regardless of the strategy, blending must be carefully controlled.
Cautions of H2. There are concerns about introducing H2 into infrastructure and equipment designed for natural gas:
- It causes embrittlement of many steel alloys used for piping.
- It escapes via leaks more readily than natural gas.
- Density differences between natural gas and H2 can cause it to separate.
- It has no natural odor, so an odorant must be added to safely detect leaks (also true for natural gas).
These concerns can be mitigated, but accommodating for the differing burning characteristics of H2 cannot be easily solved:
- Natural gas has a stoichiometric adiabatic flame temperature of 1,937°C, while H2 has a higher flame temperature of 2,182°C.
- The flame speed of natural gas is a moderate 0.4 m/sec, whereas for H2, it is 1.7 m/sec.
- On a volumetric basis, H2 has lower heat value than natural gas, so a blend loses heat value. The Wobbe index for pipeline natural gas is 1.21, but with 20% H2 content, it drops to 1.15.
The difference in temperature can usually be tolerated, but flame speed is more problematic when H2 is fed into a burner designed for natural gas—if H2 must be burned in its pure state, special burners are necessary. Burners designed for natural gas that use premix, lean premix or rapid premix designs do not perform properly with pure H2 or a high-concentration blend due to flashback. This occurs when gas velocity at the burner nozzle exit is slower than the flame speed in a premixed application. Depending on a given burner design, flashback can begin when H2 concentration reaches 30% or more, hence the general rule that H2 content must not exceed 30% for most combustion applications.
However, even if the burner resists flashback, the flame size and shape differ as H2 concentration increases. This characteristic is a particular problem with gas turbines since they are designed with very specialized burners.
Controlling H2 content. Facilities that use substantial volumes of natural gas in critical applications, such as a gas turbine, should be concerned about the composition of incoming pipeline gas since it may contain many more components than simple methane (CH4) (TABLE 1). In fact, some regulations allow for CH4 to be as low as 70% of the mix, with higher hydrocarbons making up the balance.
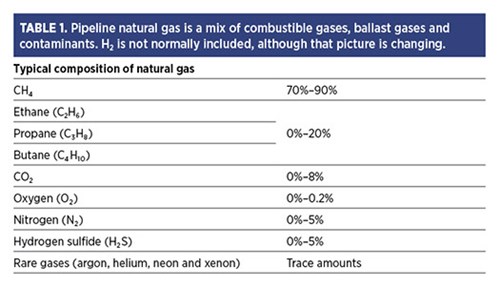
TABLE 1. Pipeline natural gas is a mix of combustible gases, ballast gases and contaminants. H2 is not normally included, although that picture is changing.
A facility wanting to do its own blending in-house using H2 generated onsite must perform real-time analysis because inlet pipeline gas may already contain H2, added upstream under utility supervision. The resulting H2 signal, which indicates composition, must be included in the larger fuel control strategy.
Traditional methods for natural gas analysis include tuned diode laser absorption spectroscopy (TDLAS) analyzers, gas chromatographs (GCs) and Raman spectroscopy. Each of these methods has strengths and weaknesses:
- TDLAS is excellent for detecting contaminants—such as water, H2S and CO2—in natural gas, but it is not the best for measuring H2 in the context of blending.
- GCs are also excellent for detailed natural gas component analysis, especially contaminants, but they cannot handle H2 concentrations approaching 10% or 20%.
- Raman spectroscopy is arguably the most practical solution for monitoring H2 content and overall composition; however, it is not suited to measure trace contaminants.
Using Raman analyzers. A primary advantage of a Raman spectroscopic analyzer (FIG. 2) is its simplicity. A probe inserted into the gas line, even at a high pressure, gathers all the necessary data, requiring no consumables and very little sample preparation. In addition, a Raman analyzer’s calibration interval is considerably longer relative to a GC. Moreover, one analyzer can support up to four independent probes, allowing measurements at multiple points through the source and blending process. The probes are connected via fiberoptic cables, so there is no power in the probes themselves. This allows them to be placed in hazardous locations while the analyzer can be up to 300 m (984 ft) away.
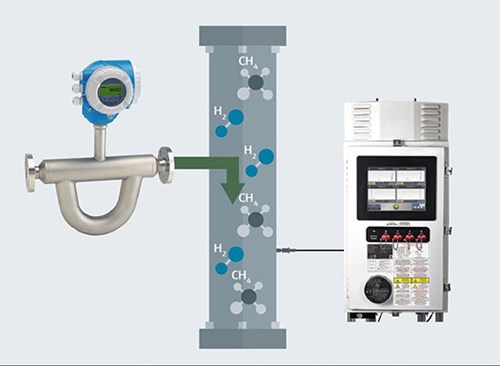
FIG. 2. Raman analyzers can measure multiple points, before and after H2 injection.
Raman analyzers are very fast and operate continuously, so they can monitor gas content with multiple measurements per minute, generating an H2 signal as a real-time process variable. This creates granular process data, suitable for control of a mixing skid, along with historization for subsequent analysis. The data can be used to meet a specific blend proportion, and these analyzers can additionally provide Wobbe index and specific gravity information, which is sometimes more useful to operators.
Injection skids. The equipment used to inject H2 into natural gas can take many forms (FIG. 3), but with common capabilities. Incoming pipeline natural gas must be evaluated by an analyzer to determine its basic composition, including any H2 that may already be blended into it. The actual blending process involves injecting H2 into the stream, controlled by pressure regulators and mass flowmeters to balance the mixture, followed by a static mixer to overcome the density differences, all assembled on a skid. Before being delivered to the final end user, the blend must be evaluated by an analyzer again to verify the final H2 proportion or Wobbe index value is as desired.
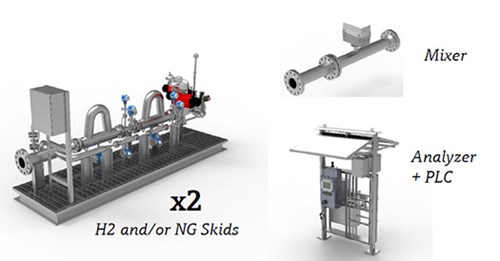
FIG. 3. Blending systems require injection, mixing and composition measurement for validation and safety.
Since pipeline natural gas can vary in composition, it is important to monitor it continuously to ensure the final blend does not drift out of spec. The incoming and outgoing blend measurements should be used to control the overall operation, hence the need for rapid measuring ability. This makes Raman analyzers ideal for the application since they can measure at multiple points simultaneously, and with much greater speed than GCs because composition is measured directly in the pipe.
Fuels for the future. While green H2 gets most of the publicity as a decarbonized energy source, it is not alone in that capacity. Other alternatives are also being evaluated, including blue H2, ammonia (NH4) offgas and syngas.
Blue H2 is made from reformed natural gas combined with carbon capture and storage, eliminating the release of any CO2. NH4 is carbonless and can be made from green H2, as well as blue H2.
Offgas and syngas are more complicated because they exist as a range of products, mostly carbon monoxide (CO) and H2 in various proportions. There is a carbon component, but it can come from a variety of sources, including biomass and waste products from other processes, rather than a direct fossil sources. Both NH4 and syngas can be burned in their pure forms or used as blending agents.
Over time, these other options may accompany green H2 on a routine basis for natural gas blending, with selection dictated by cost and availability. Consequently, any facility looking at deploying a blending skid should make sure it has the capability of handling these other gases. Fortunately, Raman spectroscopy analyzers possess the ability to measure these other components without replacing the measurement hardware, making them future-proof for other low-carbon opportunity fuels that lie ahead.
Gas turbines and fuel flexibility. A primary concern among stationary gas turbine builders and users is efficiency. These are generally high-horsepower installations that run continuously and consume large amounts of natural gas. Delivering the specified power and efficiency requires the precise type of fuel specified, so turbine operators are understandably meticulous about their supply of natural gas. A minimum Wobbe index value is usually specified to achieve name-plate performance, so substandard fuel is not tolerated.
At the same time, builders and operators are concerned about emissions, so there is also interest in alternative non-fossil fuels. The question becomes how strong a blend can be burned without sacrificing performance.
One U.S. utility company is operating a combined-cycle gas turbine power generation facility that is now burning a blend of natural gas with 5% H2 obtained as a process offgas from a nearby chemical plant. The author’s company assembled the injection and blending equipment, with proprietary flowmetersa and a proprietary Raman spectroscopy-based analyzerb (FIG. 4) to control the blending process and monitor fuel quality.

FIG. 4. The proprietary Raman analyzer shownb can provide data from four measuring points and measure multiple blending gases when needed.
Another southeastern U.S. power plant is working in cooperation with its turbine builder to reduce carbon emissions by operating with a 20% H2 blend, the first installation to validate such a high concentration on an advanced-class gas turbine in North America. This installation employs dry low NOx turbines to reduce emissions without impact on its maintenance intervals, and the unit remains in compliance with its existing air permit.
Looking into the future, turbine builders are testing new designs that are also capable of running on NH4 blends, and even 100% NH4 fuel. Other plants are using flare gas or offgas, and some of these are already operating in various locations, equipped with Raman spectroscopic analyzers to monitor and control fuel mixtures.
Programs to improve sustainability by reducing carbon emissions from fossil fuels are on the rise, and these will undoubtedly become a regular feature for equipment builders and operators. Fortunately, the measurement technologies necessary to support the safe deployment of these advances are already available.
NOTES
a Endress+Hauser’s Proline Promass Q Coriolis flowmeters
b Endress+Hauser’s Raman Rxn5 spectroscopy-based analyzer

CORY MARCON is the Power and Energy Industry Marketing Manager for Endress+Hauser USA. He is responsible for the overall business development and growth of the company position related to traditional power generation and the energy transition. As part of this role, he serves as the U.S. representative in the global SIG (Strategic Industry Group), helping develop education, the long-term vision, brand and product direction within Endress+Hauser. Marcon graduated from McGill University in 2012 and has a decade of experience in many forms of energy, including solar, wind and gas.




Comments