An evaluation of shale gas reforming technologies—Part I: Technology overview and selection
Shale gas monetization to value-added chemicals has gained extensive interest in the past decade due to the increased supply and proven reserves of shale gas.1–3 Natural gas monetization in the U.S. has not changed much despite the significant growth in shale gas production after 2010. An important pathway for utilizing and monetizing shale gas in the chemical industry is shale gas reforming to produce synthesis gas (syngas), which contains primarily hydrogen (H2) and carbon monoxide (CO).4–6
Shale/natural gas account for about half of the total global capacity share of reforming.7 The quality of the produced syngas is primarily characterized by its hydrogen:carbon molar ratio.1,8 The quality of the syngas may also vary across the different technology types of methane reforming.9 The most commonly used technologies for the commercial production of syngas from methane (CH4)—the primary components in shale/natural gas—are steam methane reforming (SMR), which represents the source of almost 70% of the H2 produced globally, autothermal reforming (ATR) and partial oxidation (POx). Dry reforming of methane (DRM) is also a promising pathway, but its commercial applications are still at the inception stages.10–12 The selection methodology presented in this work is based on the reforming of CH4 to syngas processes.
This article provides an overview of reforming technologies and shows a systematic methodology for the selection of the appropriate methane reforming technology based on the desired capacity and product type. This will enable process engineers to swiftly assess the most competitive and suitable reforming technology for a project.
BACKGROUND: TYPES OF REFORMING
Steam methane reforming (SMR). SMR is the most widely used pathway to produce syngas. The catalytic reaction between steam and methane takes place at an elevated temperature (700°C–1,100°C).13 The primary chemical reaction for SMR is shown in Eq. 1:
CH4 + H2O à CO + 3H2 ΔH0298 = 206 kJ/mol (1)
The reaction is highly endothermic; therefore, heat must be supplied for the reaction to be carried out. The H2/CO ratio of the syngas can be varied from 3 to approximately 6; this ratio may be increased/decreased by adjusting the steam-to-carbon (S/C) ratio.14 SMR is often used in ammonia (NH3) and H2 production plants, and the process configuration of these plants is slightly modified to tune the H2/CO ratio. A water-gas-shift (WGS) reactor is used after the primary reforming.
WGS reaction. The WGS reaction converts the CO in the reformer syngas to H2 and CO2 with the addition of steam. The reaction is exothermic and is shown in Eq. 2:
CO + H2O à H2 + CO2ΔH0298 = –41 kJ/mol (2)
WGS typically takes place in two subsequent reactors: a high-temperature shift (HTS) reactor and a low-temperature shift (LTS) reactor. The main difference between the two stages is the temperature at which the reaction takes place. For HTS reactors, the temperature is ~450°C, while the temperature is 250°C for LTS reactors. The H2-rich stream is then sent to a methanation step to convert the CO residuals into methane to prevent catalyst deactivation, while CO2 is removed via one of the available CO2 removal technologies.
Partial oxidation (POx). The partial oxidation (POx) of methane is a non-catalytic reaction, where oxygen is reacted with methane to form syngas and heat (Eq. 3):
CH4 + 0.5 O2 à CO + 2H2 ΔH0298 = –36 kJ/mol (3)
The produced syngas from this reaction has a low H2/CO ratio (H2/CO = 1.8–1.7). Therefore, in applications where a higher H2/CO ratio is desired, an additional source of H2 is required. One advantage of this reforming pathway is the high capacities that it can handle. For example, the Pearl GTL in Qatar utilizes two trains of the Shell Gasification Process (SGP®), and each train is capable of processing 800,000 sft3d of natural gas.15 However, this reforming pathway requires the supply of oxygen from an air separation unit (ASU). An additional H2 stream may be required to increase the H2/CO ratio to 2.0.
Autothermal reforming (ATR). Autothermal reforming falls in between SMR and POx when it comes to the H2/CO ratio and energy requirements. It utilizes both steam and oxygen to produce syngas (Eqs. 1 and 3). The H2/CO syngas ratio attained from ATR can vary between 1.8 and 3.6, depending on the ratios of steam and oxygen to methane in the feed. The heat required for the SMR reaction is typically supplied by the heat produced from the POx reaction. ATR can process a capacity that is higher than a standalone SMR. Also, ATR can be integrated with SMR in the reforming unit: this reforming configuration is referred to as two-step reforming.
Two-step reformer. Because some limitations are associated with SMR and ATR, combining these types of reforming in one configuration can increase the reforming capacity of the plant, thus increasing the product output. This approach has been widely used for methanol and gas-to-liquids (GTL) plants. The advantages of this process configuration include the ability to increase the capacity and the ease of tuning the syngas ratio by adjusting the reactants being fed into each step.
Dry reforming of methane (DRM). This reforming pathway has gained a lot of interest recently due to its huge potential in utilizing large quantities of CO2 as a primary reactant to form syngas.11 In DRM, CO2 is reacted with methane to produce an equimolar of syngas (H2/CO = 1) that can be used for applications where a stream with rich CO content is desired. The primary reaction is shown in Eq. 4:
CH4 + CO2 à CO + H2ΔH0298 = 247 kJ/mol (4)
However, the main challenge with DRM is that it is highly endothermic, meaning that it requires an external energy source to supply heat for the reaction to be carried out. Unless the heat required by the reaction is completely or partially supplied from a green/renewable energy source, the net CO2 emissions could become positive and thus emit more CO2 than what has been consumed during the reaction.16,17 Recent investigations indicate that novel process configurations involving DRM and reactors that can form coke have the ability to reduce the overall CO2 emissions from the DRM process.18
DOWNSTREAM PROCESSING PATHWAYS FOR SYNGAS
This section covers three major applications for converting syngas into value-added products that have been considered in this study: GTL, methanol and ammonia.
Fischer-Tropsch (FT) liquids/GTL. The Fisher-Tropsch process consists of three main units: methane reforming, FT synthesis, and hydrocarbon upgrading and fractionation. Based on the type of the reforming technology used, some plants may need to have an air separation unit (ASU) facility nearby to supply oxygen to the reformer. The required H2/CO ratio in the syngas for the FT reaction is around 2—this syngas ratio can be achieved through one of the various reforming technologies and designs discussed previously.
The selection of such a design is determined by multiple factors, one of which is plant capacity. For example, the ORYX GTL in Qatar and the Escravos GTL in Nigeria are typical design examples: both plants have a capacity of 34,000 bpd and utilize a standalone ATR for the production of syngas with an auxiliary ASU. Conversely, the 140,000-bpd Pearl GTL plant in Qatar, the world’s largest GTL plant, uses two trains utilizing the SGP and contains the world’s largest oxygen generation unit (ASU).6
Methanol. Methanol (CH3OH) is the simplest form of alcohol and a key intermediate to a vast number of petrochemicals and synthesis fuels. Methanol is also emerging as an alternative for existing transportation fuels since it can be produced from renewable resources.18,19 Methanol is commercially produced via the reforming of methane, and the required syngas ratio for methanol synthesis is calculated through the following expression (Eq. 5):20,21
M = (H2-CO2)/(CO+CO2) ≈ 2 (5)
Similar to the GTL process, the methanol process starts with the reforming of methane, followed by synthesis gas compression and finally methanol synthesis and purification. The technology selection for methane reforming depends on the desired capacity of the methanol plant and the available feedstock. Conventional SMR is typically selected when the capacity plant’s capacity is less than 115 MMsft3/d (3,000 tpd methanol). To achieve the desired syngas ratio using the SMR route, an additional stream of CO2 is fed to the reformer so the syngas ratio becomes around 2.
As the capacity increases, the choice of reforming technology shifts from SMR toward two-step reforming. In two-step reforming, methane is partially reformed in the SMR (first reformer), while the rest of the unreacted methane is sent to the ATR (second reformer). The co-reactants fed to each reactor are adjusted so that the appropriate syngas ratio is attained. For larger methanol plants, the suitable reforming technology in this case would be a standalone ATR.
In addition to the conventional reforming technologies, syngas may be produced through DRM that utilizes CO2 as the primary reactant with methane; however, the produced syngas must be enriched with an external H2 source to achieve the desired syngas ratio for methanol synthesis. The external H2 source could be obtained from a nearby plant or through combining DRM with conventional SMR to make up for the H2 deficiency in the syngas from DRM.
Ammonia. Ammonia is one of the most widely produced chemicals, with a global capacity of 180 MMtpy.22 Ammonia is typically used for the production of fertilizers; recently, it has emerged as an energy carrier as well as a transportation fuel. The conventional chemical route to produce ammonia is SMR followed by the Haber-Bosch process. In the reforming unit, SMR is typically followed by a WGS step to enrich the syngas content with H2 via the conversion of CO and steam to H2 and CO2, since the synthesis of ammonia requires hydrogen and nitrogen only (Eq. 6):
N2 + 3 H2 à 2NH3 (6)
As the demand for ammonia has grown over the years, the selection of the reforming technology for the new ammonia plants has changed to accommodate the rising demand, where new ammonia plants implement ATR rather than the conventional SMR for syngas production. The produced syngas from the ATR is sent to a WGS step so that the excess CO is converted to H2 in the presence of steam.
Technology selection criteria. Numerous applications in the chemical industry require the production of syngas. Each syngas project is unique when it comes to the factors that may influence the selection of the reforming route. The main factors include the desired syngas ratio (or the end-product), the available resources (e.g., a nearby H2 or oxygen source) and the capacity of the plant. In practice, the procedure of selecting the most suitable technology for a project is a complex and extensive task. However, developing a method for a rapid technology screening and selection would be of value at the early stages of the project.
FIG. 1 shows an overview of the characteristics of available commercial methane reforming technologies. The graph provides a quick overview and reference for process engineers and decision-makers to help assess and narrow down the potential reforming pathways based on the available resources and the project requirements. The typical capacity ranges from 6 MMsft3d–800 MMsft3d, and the H2/CO ratio ranges from 1.6–8
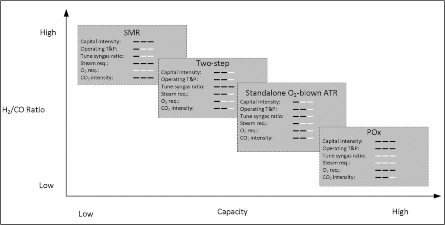 |
| FIG. 1. The most common commercial reforming pathways and their key characteristics. |
In Part 2, the development of the order-of-magnitude correlations for cost and CO2 emissions of methane reforming will be presented. A case study will be shown to illustrate the use of the correlations. GP
LITERATURE CITED
- Al-Douri, A., D, Sengupta and M. M. El-Halwagi, “Shale gas monetization—A review of downstream processing to chemicals and fuels,” Journal of Natural Gas Science and Engineering, Vol. 45, September 2017.
- Elbashir, N. O., M. M. El-Halwagi, I. G. Economou and K. R. Hall, Natural gas processing from midstream to downstream, Wiley, February 2019.
- Al‐Douri, A., A. S. Alsuhaibani, M. Moore, R. B. Nielsen, A. A. El‐Baz and M. M. El‐Halwagi, “Greenhouse gases emissions in liquified natural gas as a marine fuel: Life cycle analysis and reduction potential,” The Canadian Journal of Chemical Engineering, July 2021.
- Noureldin, M. M., N. O. Elbashir and M. M. El-Halwagi, “Optimization and selection of reforming approaches for syngas generation from natural/shale gas,” Industrial & Engineering Chemistry Research, Vol. 53, Iss. 5, ACS Publications, February 2014.
- Martinez-Gomez, J., F. Nápoles-Rivera, J. M. Ponce-Ortega and M. M. El-Halwagi, “Optimization of the production of syngas from shale gas with economic and safety considerations,” Applied Thermal Engineering, August 2016.
- Klerk, A. d., Fischer-Tropsch refining, 1st Ed., John Wiley & Sons, 2012.
- Khan, H., “Global syngas overview,” Stratas Advisors, 2019, online: https://www.slideshare.net/StratusAdvisors/statas-advisors-global-syngas-overview-by-dr-habib-khan
- Song, X. and Z. Guo, “A new process for synthesis gas by co-gasifying coal and natural gas,” Fuel, Vol. 84, March2005.
- Alsuhaibani, A. S., S. Afzal, M. Challiwala, N. O. Elbashir and M. M. El-Halwagi, “The impact of the development of catalyst and reaction system of the methanol synthesis stage on the overall profitability of the entire plant: A techno-economic study,” Catalysis Today, Vol. 343, March 2020.
- Afzal, S., D. Sengupta, A. Sarkar, M. El-Halwagi and N. Elbashir, “Optimization approach to the reduction of CO2 emissions for syngas production involving dry reforming,” ACS Sustainable Chemistry & Engineering, April2018.
- Challiwala, M. S., M. M. Ghouri, P. Linke, M. M. El-Halwagi and N. O. Elbashir, “A combined thermo-kinetic analysis of various methane reforming technologies: Comparison with dry reforming,” Journal of CO2 Utilization, Vol. 17, January 2017.
- Noureldin, M. M., N. O. Elbashir, K. J. Gabriel and M. M. El-Halwagi, “A process integration approach to the assessment of CO2 fixation through dry reforming,” ACS Sustainable Chemistry & Engineering, March 2015.
- Speight, J. G., Handbook of industrial hydrocarbon processes, 2nd Ed., Gulf Professional Publishing, 2019.
- Rostrup-Nielsen, J. R., J. Sehested and J. K. Nørskov, “Hydrogen and synthesis gas by steam- and CO2 reforming,” Advances in Catalysis, January 2002.
- “Pearl GTL plant running at reduced production,” Hydrocarbon Processing, 2016.
- El-Halwagi, M. M. and J. E. Campbell, “An integrated and tunable system for the production of syngas and chemicals via solar-assisted electrolysis and combined reforming,” WO2019147786, 2021.
- Tavasoli, A. and G. Ozin, “Green syngas by solar dry reforming,” Joule, Vol. 2, Iss. 4, April 2018.
- Challiwala, M. S., H. A. Choudhury, D. Wang, M. M. El-Halwagi, E. Weitz and N. O. Elbashir, “A novel CO2 utilization technology for the synergistic co-production of multi-walled carbon nanotubes and syngas,” Scientific reports, 2021.
- Räuchle, K., L. Plass, H. Wernicke and M. Bertau, “Methanol for renewable energy storage and utilization,” Energy Technology,2016.
- Rostrup-Nielsen, J. and L. J. Christiansen, Concepts in syngas manufacture, Imperial College Press, London, June 2011.
- Hernandez-Perez, L. G., A. S. Alsuhaibani, N. Radwan, M. M. El-Halwagi and J. M. Ponce-Ortega, “Structural and operating optimization of the methanol process using a metaheuristic technique,” ACS Sustainable Chemistry & Engineering, Vol. 8, 2020.
- David, W., “Ammonia: zero-carbon fertiliser, fuel and energy store,” Policy Briefing, The Royal Society, 2020.
 |
ABDULRAHMAN S. ALSUHAIBANI holds a PhD in chemical engineering from Texas A&M University. His research interest is in the area of sustainable design of industrial systems, including the production of low-carbon alternative fuels. Prior to earning his PhD, Dr. Alsuhaibani worked for Saudi Aramco R&DC.
 |
SHAIK AFZAL is a Principal Engineer in the Hydrogen Technology Center at GTI Energy. He is involved in projects for low-carbon production and the utilization of hydrogen for various downstream applications. Dr. Afzal’s past research experience is in the areas of heterogeneous catalysis, process simulation and optimization, and techno-economic analyses. He worked for 2 yr in oil refinery operations and process safety engineering in India and Qatar. He earned his B.Tech degree in chemical engineering from RV College of Engineering in India and received his MS degree and PhD in chemical engineering from Texas A&M University. Dr. Afzal did his postdoctoral training at the National Renewable Energy Laboratory.
 |
NIMIR ELBASHIR is a joint professor of Chemical Engineering and Petroleum Engineering at Texas A&M University at Qatar and the Director of Texas A&M’s Gas & Fuels Research Center.He joined Texas A&M in 2008 from the BASF Catalysts R&D department. Elbashir has more than 20 yr of research and technology development experience in catalysis, reaction engineering, fuel processing and reactor design. He has more than 10 U.S. and international patents issued in his name, including the patents for the CARGEN® technology and numerous publications in the form of peer-reviewed journal papers, books, chapters and industry reports. He has established and led many multi-million-dollar unique academia-industry collaborations, and has received numerous awards recognizing his research activities.
 |
MAHMOUD EL-HALWAGI is a professor and holder of the Bryan Research and Engineering Chair and the Managing Director of the Gas and Fuels Research Center at Texas A&M University. Dr. El-Halwagi’s main areas of expertise are sustainability, process integration, synthesis, design, operation and optimization. He is the author of three textbooks, the co-author of more than 500 referenced papers and book chapters, and the co-editor of 10 books. Dr. El-Halwagi is the recipient of several awards, including the AIChE Computing in Chemical Engineering Award and the AIChE Sustainable Engineering Forum Research Excellence Award. He earned his BS and MS degrees from Cairo University and his PhD from the University of California, Los Angeles.




Comments