Maintain temperatures in gas sample lines to enhance measurement accuracy
R. Rieken, Swagelok Co., Solon, Ohio
Within a gas sampling system, numerous variables can affect the accuracy of analyzer measurements. However, the most common culprit of incorrect measurements is the improper control of sample line temperatures. When a hot gas sample is pulled from a process line into a sampling system that is unheated or at a too-low temperature, the gas will cool rapidly. As it does, it will naturally condense, creating a sample that is part gas and part liquid. The concentration of the remaining gas in the sample will therefore no longer be representative of the process gas, and the analyzer measurement will not provide a true reading of process conditions.
To prevent a gas sample from changing phases, system designers and operators must maintain appropriate temperatures throughout the sampling system. Doing so will help the sample remain representative of the process fluid. Operators may still encounter problems that lead to inaccurate measurements. Fortunately, a few symptoms can indicate an issue with the line temperature, including the presence of volatile liquid in sample lines, the formation of oily liquid in sample lines and the occurrence of incorrect analyzer measurements.
This article reviews each of these symptoms to help plants and laboratories better maintain the required temperature for gas samples to realize more accurate analyzer results.
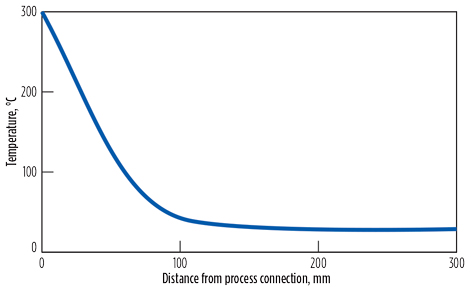 |
|
FIG. 1. When 300 C process gas meets uninsulated tubing, its temperature drops drastically, cooling to 30 C in about 120 mm of bare tubing. Image adapted from Gross and Bundschuh.1 |
Avoid volatile liquid in sample lines. Condensation occurs when the hot gas from the process meets cold metal within the sampling system. The gas cools rapidly to the temperature of the metal. For example, Fig. 1 shows how hot gas from a furnace cools from 300°C to 30°C in the time it takes to travel through about 120 mm of bare tubing. Even when the tubing is insulated, the gas cools to the ambient temperature after traveling through approximately 0.5 m of tubing (Fig. 2). The only way to stop the gas from cooling is to heat the tubing to the desired temperature.
With condensation often viewed as a nuisance, system designers and operators typically focus on ways to remove the liquid from sample lines using devices such as coalescers, kinetic separators or catchpots. Removing the liquid in this manner can be a valid approach. However, doing so only cures the symptom of condensation and not the root cause.
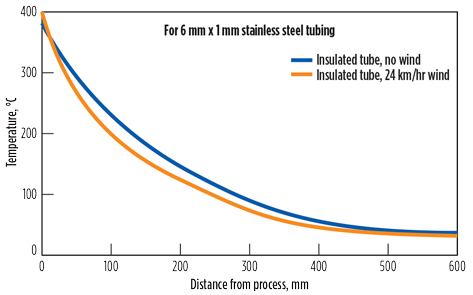 |
|
FIG. 2. With insulated tubing, hot process gas will cool to the ambient temperature after only 0.5 m of tubing. Image adapted from Gross and Bundschuh.1 |
Instead of removing the condensate, it is better to prevent condensation and maintain dry gas sample lines because condensation is more than a nuisance. It can change the analysis of the sample in one of two ways:
- If the condensate does not dissolve the analyte, then the remaining gases will have a higher concentration than the original gas sample.
- If the condensate partly dissolves the analyte, then the sample will fractionate into a liquid and vapor having different compositions, neither of which represents the process composition.
When the condensate does not dissolve the analyte, it is acceptable to remove the condensate before gas enters the sample line. The system first cools the gas and then removes the condensate, using a collection device, before allowing the gas to enter the sample line. Alternatively, heating the entire sample line and controlling its temperature at 20° above the maximum dewpoint of the sample will prevent the development of condensate altogether.
For example, when the sample’s dewpoint is 100°C, a sample line temperature of at least 120°C should be maintained. This 20° margin is necessary to account for inevitable cold spots in the line. To reduce the dewpoint temperature—and, therefore, reduce the required sample line temperature—the sample gas should be adjusted to the lowest workable pressure.
When the condensate will only partly dissolve the analyte, the sample will be ruined and cannot be used for analysis. Whenever a soluble vapor and its condensate are both present anywhere in a sampling system (even in the process pipe), the vapor and liquid compositions differ, and neither represents the original sample composition. Even worse, the problem cannot be corrected within the sampling system to achieve an accurate analysis.
For example, the system can separate and remove the liquid to allow a dry vapor sample to reach the analyzer; however, the composition of that vapor will no longer represent the process conditions. The system designer could try to pass the two-phase sample through a vaporizing regulator or by heating the line to revaporize the liquid. Unfortunately, though, the sample will have become irretrievably fractionated, with a new composition that is unrelated to the current process.
Prevent oily liquid in sample lines. Finding a nonvolatile liquid—one that will not readily evaporate into a gas under existing conditions—in a sample line is a different issue, because increasing the line temperature will not help. In fact, a hotter sample line may exacerbate this problem.
The liquid oil may come from the process, or it may form in the line itself. For example, olefin samples often produce a green oil, which is a polymer that forms due to an ongoing polymerization reaction in the gas. Increasing the sample line temperature will accelerate the rate of oil formation and make the problem worse. The solution is to run the line at the lowest workable pressure and temperature while also removing the oil by installing a coalescer and a drain at both ends of the line.
When vaporizing a liquid sample, a coalescer and a drain should be installed immediately after the vaporizer to prevent nonvolatile oils dissolved in the liquid from entering the sample line. It is essential to remove any oil before it enters the transport line because an internal coating of oil in a transport line acts like a chromatograph column, separating gases due to differing adsorption rates as they flow through the line.2 In this situation, the gases in the sample will each have different retention times as they travel down the wet line. If the analyzer is measuring several gases, each measurement will come from a different time in the process, which will thoroughly confuse operators and skew control protocols.
Pay attention to incorrect measurements. The first indication of not maintaining the appropriate temperature of a gas sample line will likely be an incorrect analyzer measurement. Such measurements may indicate that the sample is cooling down, that permeation is occurring, that condensation is present or another issue.
If the gas sample line is cooling, adsorption may occur—a problem that results in low ppm concentrations of polar gases like water vapor and hydrogen sulfide sticking to the tubing walls. Applying more heat to the line may provide some improvement. A better solution is to use electropolished tubing or tubing that has an ultrathin layer of silicon applied to its interior. The resulting smooth surfaces discourage adsorption of the analyte gases.
Excessive heat in a gas sample line can increase permeation, especially in polymer tubing. With permeation, molecules from the sample line pass through the tubing wall, escaping into the atmosphere and altering the concentration of the sample compared with its original form in the process line. Oxygen or moisture from the atmosphere may also enter the tubing, further skewing the sample composition. To avoid permeation, it may help to reduce the temperature of the sample. Better yet, the polymer tubing can be replaced with silicon-treated metal tubing. A single run of tubing with the minimum number of tube unions can be used to reduce permeation potential.
Condensation always affects sample composition—and, therefore, analyzer measurements—because it removes molecules from the gas phase. Condensate should be removed before a gas sample is transported to the analyzer location, thereby ensuring that the transport line is dry. After removing the condensate, the sample pressure should be reduced or the line should be heated to desaturate the gas.
Maintain the heat. A primary key to ensuring accurate gas sample analyzer measurements is to prevent the presence of condensation in sample lines by maintaining appropriate temperatures throughout the system. However, using either uninsulated or insulated tubing will not be sufficient. Instead, heat the entire sample line and maintain the recommended 20° temperature differential between the sample and its maximum dewpoint temperature.
A prefabricated tube bundle is by far the best option for maintaining temperature to minimize condensation. Such assemblies of one or more tubes, a heat tracing line and an insulating material are fabricated similarly to electrical cables, featuring a waterproof, extruded-polymer casing. Prefabricated bundles are available in long, continuous lengths, which reduces the number of tubing unions within a system. Reducing those connection points is critical, as tubing unions are notorious cold spots where condensation is likely to form.
Even when sample lines are heated, issues that can lead to inaccurate analyzer measurements may still arise. Signs of volatile or oily liquid in sample lines should be heeded, and the occurrence of incorrect analyzer measurements should be observed to determine whether any variables must be adjusted within the system, including the sample line heat. GP
Note
This article was adapted from Industrial Sampling Systems: Reliable Design and Maintenance for Process Analyzers, a process sampling textbook authored by Tony Waters and published by Swagelok Co. in 2013.
Literature cited
- Gross, W. J. and J. E. Bundschuh, “Best practice considerations for the specification, design, installation, and maintenance of process and emissions analyzer sample transport systems,” Proceedings of the 53rd Annual Spring Symposium, Calgary, Alberta, Canada, April 2008.
- Jackson, R. G., “Sample system speed of response,” Proceedings of the 1961 National Symposium on Instrumental Methods of Analysis, Vol. 7, Instrument Society of America, Pittsburgh, Pennsylvania, 1961.
 |
Randy Rieken is Market Manager for chemical and refining for Swagelok Co. He is responsible for the development and implementation of chemical and refining market-driven strategies. Mr. Rieken joined Swagelok in 2014 and has more than 25 yr of global business development experience providing engineered fluid control components, subsystems and instrumentation solutions for the most critical applications in the oil and gas, chemical, semiconductor, power and life sciences markets.




Comments